When a product leaves a manufacturing facility, it should be safe. Not just legally safe, but actually safe. That’s not luck. It’s the result of a quiet, systematic process called environmental monitoring. This isn’t about checking the final product after it’s made. It’s about catching contamination before it ever gets there. In food plants, pharmaceutical labs, and cosmetic factories, this process is the first and most important line of defense against illness, recalls, and brand damage.
What Environmental Monitoring Actually Does
Environmental monitoring isn’t just swabbing surfaces and hoping for the best. It’s a science. It’s about testing air, water, and surfaces for things that shouldn’t be there: bacteria like Listeria or Salmonella, mold spores, metal particles, chemical residues, even tiny bits of dust. These aren’t just theoretical risks. In 2022, foodborne illnesses tied to poor environmental controls cost the U.S. economy $77.7 billion. That’s not a number. That’s hospitals, lost workdays, and families affected. The goal? Stop contamination at the source. The CDC says environmental monitoring is one of the few ways to confirm a hazardous condition is under control. If you’re making ready-to-eat meals, pharmaceuticals, or skincare products, your environment is part of the product. A single mold spore on a conveyor belt can ruin a batch. A drop of contaminated water in a sterile vial can cause an infection. Monitoring doesn’t just catch problems-it prevents them.The Zone System: How Facilities Map Risk
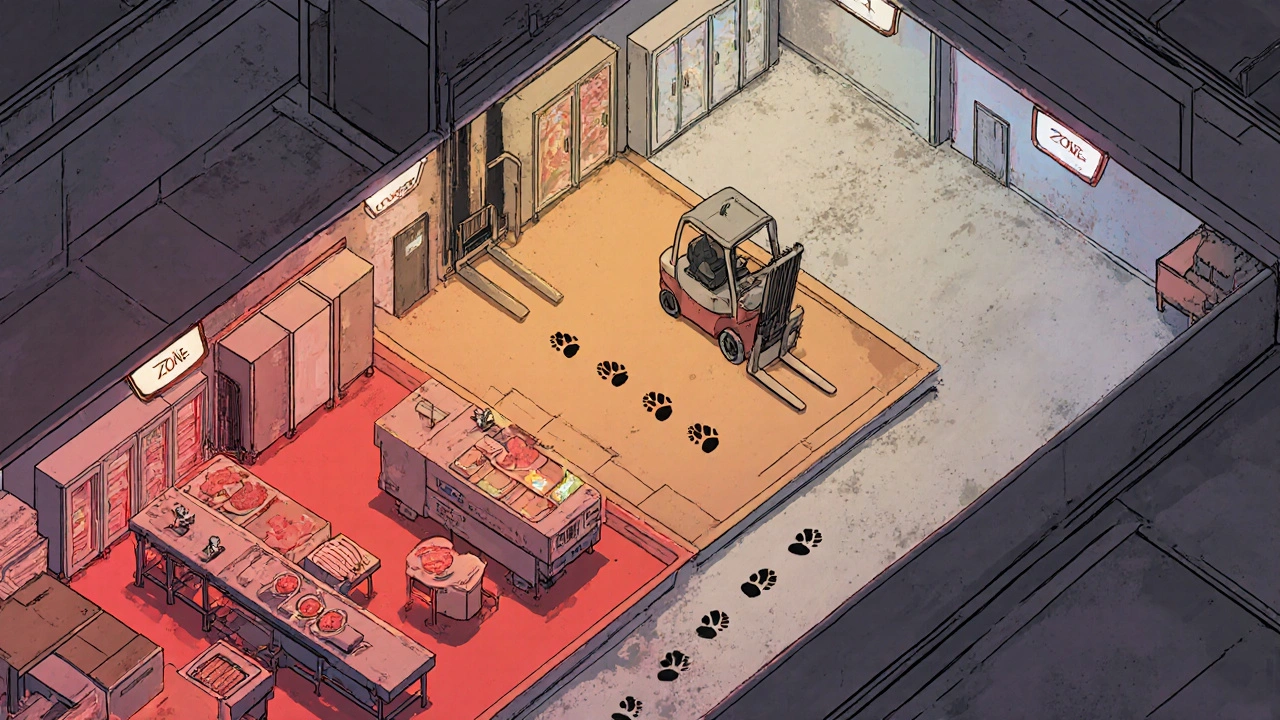
Every facility that does serious environmental monitoring uses a zone system. It’s simple, practical, and universally adopted. Zones are ranked by risk, from highest to lowest.- Zone 1: Direct food or product contact surfaces. Think slicers, mixers, filling nozzles, packaging rollers. These are sampled daily or weekly. A single Listeria cell here can end up in a sandwich or a vial of medicine.
- Zone 2: Surfaces near Zone 1 but not in direct contact. Equipment housings, refrigeration units, nearby walls. These are tested weekly to monthly. Condensation dripping from an overhead pipe in Zone 2 can contaminate a Zone 1 surface. That’s why some facilities treat overhead pipes as Zone 1 if they drip.
- Zone 3: Remote surfaces in the production area. Forklifts, carts, floor edges, utility lines. These are tested monthly. Surprisingly, a 2010-2013 study by PPD Laboratories found that floors were the source of 62% of all contamination alerts-even though they’re Zone 3. People forget how much gets tracked in.
- Zone 4: Areas outside production. Break rooms, hallways, storage areas. Tested quarterly. Low risk, but still monitored. A worker sneezing in Zone 4 and then walking into production? That’s how outbreaks start.
How Testing Works: Tools and Methods
Different contaminants need different tools.- Microbial testing: Swabs, sponges, or air samplers collect samples from surfaces or air. Results come back in 24-72 hours as colony-forming units per cubic meter (CFU/m³). This is the gold standard for detecting Listeria, Salmonella, and mold.
- ATP testing: This isn’t a microbial test. It measures adenosine triphosphate, a molecule found in all living cells. It gives results in seconds. Facilities using ATP see 32% faster production turnarounds because they don’t wait days for lab results. But ATP doesn’t tell you what’s there-just that something organic is present. It’s a quick check, not a final answer.
- Air sampling: Liquid impingers and solid impactors pull large volumes of air through devices. Slit or sieve designs must be sterilized before each use. If the sampler itself is dirty, you’re just contaminating your own test.
- Water testing: Pharmaceutical facilities use total organic carbon (TOC) and conductivity tests to meet USP <645> standards. Food plants check for EPA compliance on municipal water.
- Chemical and metal detection: Gas chromatography (GC), HPLC, and ICP (Inductively Coupled Plasma) detect traces of solvents, pesticides, or heavy metals. These are critical in cosmetics and high-purity manufacturing.
Regulations: Why This Isn’t Optional
This isn’t a best practice. It’s a legal requirement.- Pharmaceuticals: EU GMP Annex 1 (updated August 2023) demands continuous air monitoring in cleanrooms. Air must meet ISO Class 5 standards-equivalent to a hospital operating room. The FDA requires the same for sterile products.
- Food: The USDA’s Listeria Rule (9 CFR Part 430) forces RTE food plants to test Zone 1 surfaces weekly for Listeria monocytogenes. The FDA’s Food Safety Modernization Act (FSMA) expanded this to all high-risk facilities.
- Inspections: FDA investigators don’t just check labels. They swab floors, drains, and equipment housings. If they find Listeria in Zone 2 or 3, they can shut you down. In 2023, 68% of facilities cited for violations had inconsistent sampling techniques.
What Goes Wrong-and How to Fix It
Most failures aren’t technical. They’re human.- Training gaps: The FDA recommends 40 hours of hands-on training before someone can collect samples. But many small facilities skip this. A worker using a non-sterile swab? You’ve just added contamination to your test.
- Inconsistent zones: As Dr. Laurel Dunn from the University of Georgia points out, one facility’s Zone 1 is another’s Zone 3. Risk assessments must be documented and reviewed quarterly. Don’t assume everyone agrees.
- Ignoring Zone 3 and 4: Floors, carts, and forklifts are where 62% of contamination events start. Cleaning Zone 1 daily means nothing if your workers track dirt in from Zone 4.
- Data silos: If your ATP results, microbiology reports, and allergen tests live in different spreadsheets, you’re flying blind. Integration is the next frontier.
The Future: AI, Real-Time Data, and Faster Results
The next wave isn’t about bigger labs. It’s about faster answers.- Next-generation sequencing (NGS): Instead of waiting 72 hours to identify a pathogen, NGS can do it in under 24 hours. The FDA is pushing for adoption.
- Real-time air monitoring: EU Annex 1 now requires continuous data trending. Sensors track particles and humidity 24/7. Alerts trigger automatically if levels spike.
- AI-powered analytics: By 2027, 38% of monitoring systems will use AI to spot patterns-like a spike in mold after a maintenance shift or a recurring hotspot near a drain. This turns monitoring from reactive to predictive.
What You Need to Start
If you’re setting up a program:- Map your zones. Document why each surface is in its zone. Get management sign-off.
- Choose your tests. Use ATP for quick checks. Use swabs and air samplers for regulatory compliance.
- Train your team. 40 hours isn’t optional. Practice swabbing. Practice sterilizing samplers.
- Integrate your data. Use one system to track ATP, microbiology, and cleaning logs.
- Test Zone 3 and 4. Don’t ignore the floor. Don’t ignore the forklift.
- Review quarterly. Adjust zones. Update methods. If nothing changed, you’re not learning.
Final Thought: It’s Not About Passing an Inspection
Environmental monitoring isn’t a box to check. It’s a culture. It’s about asking: “What could go wrong before this product leaves here?” And then doing something about it. The most successful facilities don’t have the most expensive gear. They have the most consistent habits. They don’t wait for an outbreak. They don’t wait for an FDA warning. They test. They clean. They learn. And they never assume it’s fine.What is the main purpose of environmental monitoring in manufacturing?
The main purpose is to detect and prevent contamination from air, surfaces, or water before it affects the product. This protects consumers from illness, avoids costly recalls, and ensures compliance with FDA, USDA, and EU regulations. It’s about stopping problems before they happen, not fixing them after.
What are the four zones in environmental monitoring?
Zone 1 includes direct product contact surfaces like slicers and filling nozzles. Zone 2 includes nearby surfaces like equipment housings and refrigeration units. Zone 3 covers remote surfaces in production areas like floors and forklifts. Zone 4 includes areas outside production, like break rooms and hallways. Risk decreases from Zone 1 to Zone 4, but contamination often starts in Zone 3 or 4.
How often should environmental sampling be done?
Frequency depends on the zone and industry. Zone 1 surfaces are tested daily to weekly. Zone 2 is sampled weekly to monthly. Zone 3 and 4 are tested monthly to quarterly. FDA requires weekly Listeria testing in Zone 1 for ready-to-eat food facilities. Pharmaceutical cleanrooms require continuous air monitoring.
What’s the difference between ATP testing and microbiological testing?
ATP testing detects organic material (like cells) in seconds and is used for quick sanitation checks. It doesn’t identify what’s there. Microbiological testing takes 24-72 hours but identifies specific pathogens like Listeria or Salmonella. ATP is a screening tool; microbiological testing is the regulatory standard.
Why do some facilities fail environmental monitoring inspections?
The top reasons are inconsistent zone classification, poor sampling technique (like using non-sterile swabs), lack of staff training, and failure to integrate data from ATP, microbiology, and allergen tests. Sixty-eight percent of facilities cite inconsistent sampling as a major issue.
Is environmental monitoring required by law?
Yes. In the U.S., the FDA enforces it under FSMA for food, and under GMP rules for pharmaceuticals. The USDA’s Listeria Rule (9 CFR Part 430) mandates weekly testing for ready-to-eat foods. The EU’s Annex 1 requires continuous air monitoring for sterile drug production. Non-compliance can lead to shutdowns, recalls, or fines.





Emma louise
November 28, 2025 AT 05:12Oh great. Another post pretending like this stuff isn't just corporate theater to justify $200 swabs and $50k air monitors. You think Listeria's some mysterious demon? Nah. It's the same dirt your grandma didn't wash off her hands before making pickles. Now we need AI and NGS just so some VP can say 'we're proactive' while the floor techs get paid minimum wage to wipe the same conveyor belt for 12 hours straight. Wake up. This isn't science. It's liability insurance dressed in lab coats.
Alex Hess
November 29, 2025 AT 06:39Zone 3 being responsible for 62% of contamination? That’s not a finding-it’s an indictment of management’s incompetence. If your entire production floor is a petri dish because you can’t afford to seal the cracks or train your staff, then no amount of ATP testing will save you. You’re not monitoring the environment-you’re documenting your own failure.
Lauren Zableckis
November 30, 2025 AT 02:09I’ve worked in three different food plants and one pharma facility. The zone system works-but only if leadership actually listens to the people doing the swabbing. The real problem isn’t the tools or the tech. It’s that the person who cleans the forklift gets zero say in where the zones are drawn. Fix the hierarchy before you fix the swabs.
Savakrit Singh
November 30, 2025 AT 16:52Kindly note that the global environmental monitoring market is projected to reach USD 12.5 billion by 2027, with pharmaceuticals contributing 42% of the revenue stream. This indicates a structural demand for compliance-driven instrumentation, particularly in ISO Class 5 environments. However, the integration gap between ATP and microbiological data remains a critical bottleneck, as evidenced by the 37% non-alignment rate reported by IDFA. Strategic investment in centralized LIMS platforms is recommended to mitigate data silos and enhance predictive analytics capabilities.
Cecily Bogsprocket
December 2, 2025 AT 16:14It’s heartbreaking how much of this is about fear instead of care. People don’t want to get sick. Families don’t want to lose someone because a drain wasn’t cleaned right. But we’ve turned safety into a checklist game instead of a culture of responsibility. The real hero isn’t the AI or the sensor-it’s the person who stays late to re-sterilize a sampler because they know someone’s life might depend on it. We need to honor that, not just track it.
Jebari Lewis
December 2, 2025 AT 21:05Wait-so ATP gives results in seconds but doesn't tell you what it is? That's like having a smoke alarm that goes off every time you toast bread. Why are we relying on this as a primary tool? And why are facilities not cross-referencing it with actual cultures? The data disconnect here is staggering. Someone needs to build a dashboard that auto-links ATP spikes to lab results. I'd volunteer to prototype it if anyone's listening.
Asha Jijen
December 3, 2025 AT 02:14Allison Turner
December 4, 2025 AT 01:09Let’s be real. This entire system exists because someone got sued in 2008. Now it’s a $12 billion industry built on fear and bureaucracy. You don’t need NGS to prevent contamination-you need managers who don’t cut corners. You need workers who aren’t terrified to speak up. You need culture. Not sensors. Not swabs. Not AI. Just decency. And we’ve lost that somewhere between the compliance forms and the quarterly audits.
Darrel Smith
December 5, 2025 AT 18:04This is how we kill small businesses. You think a family-owned deli can afford a $10,000 air sampler? No. So they get shut down by the FDA because a single mold spore was found on a wall they didn’t even know was a Zone 2. Meanwhile, big corporations pay consultants to game the system and get waivers. This isn’t safety-it’s a weapon. And the little guy? He’s the one who pays with his livelihood. Wake up. This isn’t about health. It’s about control.
Iives Perl
December 7, 2025 AT 11:50AI monitoring? Real-time sensors? LOL. They’re just tracking your movements. Every time you walk near a sensor, they log your bio-data. They’re building a database of every worker’s sweat, breath, and skin cells. This isn’t contamination control. It’s surveillance capitalism in lab coats. The FDA doesn’t care about your health-they care about your biometrics. Don’t believe me? Just wait till they start charging you for ‘exposure credits’.
sharicka holloway
December 8, 2025 AT 17:27My cousin works in a small cosmetic lab. They don’t have Zone 1, 2, 3-they have ‘clean’ and ‘not clean.’ But they’ve never had a recall. Why? Because everyone knows each other. The guy who mops the floor knows the chemist who mixes the cream. They talk. They fix things before they become problems. Maybe the answer isn’t more tech. Maybe it’s more humanity.
Edward Batchelder
December 9, 2025 AT 15:01What strikes me most is how this system, flawed as it is, saves lives. I’ve seen the reports-families who didn’t lose a child to Listeria because a swab caught it before the product shipped. That’s not bureaucracy. That’s grace. Let’s fix the gaps, sure. But let’s not throw out the system because it’s imperfect. The alternative? A world where people get sick because we got lazy. That’s a cost we can’t afford to pay.
reshmi mahi
December 10, 2025 AT 00:05laura lauraa
December 10, 2025 AT 09:46And yet… who gets to decide what ‘contamination’ even means? Who decides if a mold spore is ‘dangerous’ or ‘harmless’? Who funded the studies? Who owns the patents on the swabs? Who profits from the fear? You talk about Listeria like it’s a monster… but the real monster is the system that profits from your terror. You’re not protecting your family-you’re feeding the machine. And you don’t even realize it.
Gayle Jenkins
December 10, 2025 AT 17:59I’ve trained over 200 people on swabbing techniques. The most important thing I teach? Don’t just follow the checklist. Ask why. If you don’t understand why Zone 2 matters because of that dripping pipe, you’re just moving a sponge around. When people get it-they don’t need to be told to clean. They do it because they care. That’s the real win. Not the data. Not the audit. The human.